
CHLORINE WATER AND POTASSIUM BROMIDE
A Halide Displacement Reaction.
Chlorine water added to a 2.0M solution of potassium bromide yields a deep orange color characteristic of molecular bromine.

MAGNESIUM BURNING IN AIR: AN EXOTHERMIC REACTION
Producing White Flame & Smoke Of Magnesium Oxide
Magnesium, a group IIA alkaline earth metal burns in air with a bright white flame, producing a white smoke of solid magnesium oxide. What remains after burning is solid magnesium oxide.
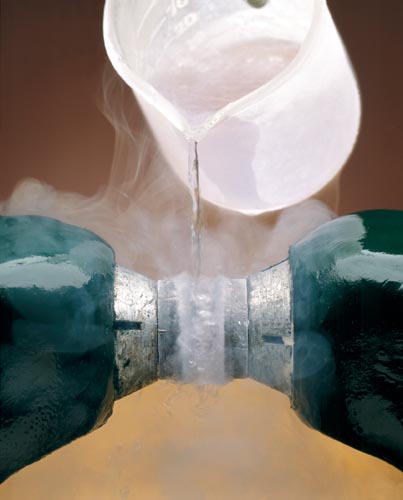
PARAMAGNETISM OF LIQUID OXYGEN
Liquid O2 Poured Between Poles of a Magnet.
Because the O2 is paramagnetic (contains two unpaired electrons) it is attracted into the magnetic field and forms a bridge between the magnetic poles.

CHEMILUMINESCENCE: LUMINOL REACTION
Cold Light Produced By A Chemical Reaction.
This oxidation reaction, catalyzed by iron, produces 5-aminophthalic acid with electrons in an excited state. Excess energy, freed as photons, is visible as blue light and useful to forensics to detect iron compounds in blood .

CHEMICALS MEASURED FOR CHEMICAL REACTIONS
A Burette Measures By Each Drop.
Other glassware include volumetric flasks & graduated cylinders.

STEEL WOOL BURNING IN OXYGEN
Placed in Flask of O2 with Tongs.
Combustion is more rapid in the presence of pure oxygen.

PHOSPHORUS (WHITE) BURSTS INTO FLAMES
Exothermic Reaction Between Oxygen and Phosphorus.
Finely divided white phosphorus, deposited on a piece of filter paper by evaporation of a carbon disulfide solution of P4, bursts into flames on contact with air.

COMPLEX ION FORMATION: NH3(LIGAND) ADDED TO NiCl2
NiCl2 & Ni(NH3)6Cl2 Solutions in Beakers.
Pale green Nickel 2+ ion reacts as a Lewis acid with the addition of the ligand (NH3)2- to form the deep blue coordination compound Ni(NH3)6Cl2, which contains the complex ion [Ni(NH3)6]2+. This process can be reversed by the addition of H+.

SOAP CURD
Hard Water Ions React With Soap To Form Curd.
Curd is a gray, slimy substance that adheres to clothes.

DRY ICE (SOLID CO2) SUBLIMATES & ACIDIFIES WATER
CO2(s) Dropped In Water Forms Carbonic Acid
CO2(s) dropped in water with bromothymol blue indicator forms carbonic acid which changes the indicator color to yellow. Addition of ammonia causes color change to blue. White smoke is a mist of water droplets condensed from the air by cold CO2 gas.
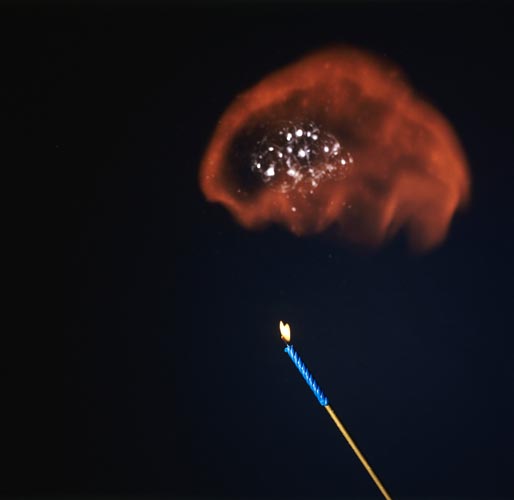
IGNITION OF HYDROGEN SOAP BUBBLES
Exothermic Reaction Between Hydrogen & Oxygen.
As the bubbles float upward, they are ignited using a candle on a long pole. The orange flame is due to the reaction of hydrogen with the oxygen in the air.
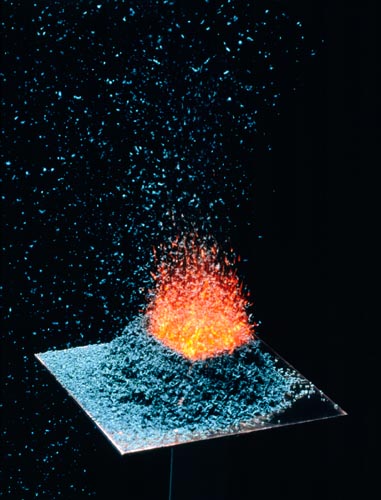
AMMONIUM DICHROMATE OXIDATION -VOLCANO EFFECT
An Exothermic Reaction.
Ammonium Dichromate(VI), (NH4)Cr2O7 crystals are ignited resulting in an exothermic reaction with end products of solid chromium (III) oxide, nitrogen gas & water vapor.

PHOSPHORUS MOON DEMONSTRATION (CloseUp)
Combustion of Red Phosphorus in An O2 Atmosphere.
The submerged phosphorus flame rises above the solution as combustion continues to draw the solution up. The product of combustion has resulted in Phosphoric acid which turned the solution red (pH=4).

CONVECTION PATTERN IN HEATED WATER
Conduction Between Solid Surface & Moving Liquid.
A container filled with water is heated by a Bunsen burner at the bottom left and cooled by ice at the top right. Dye added over the ice travels in a circular direction demonstrating heat transfer by the circulation of currents from one region to another

COPPER METAL REACTS WITH NITRIC ACID
Copper Metal In A Beaker Of Nitric Acid
Oxidation of copper metal by nitric acid produces nitric oxide (NO) gas which is immediately oxidized in air to form brown nitrogen dioxide (NO2) gas. The copper atoms lose two electrons to form Cu2+ ions which give a blue color in water.

PROPERTIES OF GASES- NITROGEN DIOXIDE
Gas Has No Definite Form Or Volume.
When the stopper is removed, the gas seeks equilibrium by filling tne larger space now available.

COPPER HEATED TO FORM COPPER II (CUPRIC) OXIDE
During Heating
When copper metal is heated in the presence of oxygen it oxidizes to form black cupric oxide.

SODIUM REACTS WITH BROMINE TO FORM SODIUM BROMIDE
Alkali Metal Reacts with Halogen Gas In Flask.
Sodium(s) is dropped into bromine(g) creating a violent exothermic reaction. The resulting ionic compound forms a stable array known as a lattice. 2Na(s) + Br2(g) NaBr(s)

REACTION BETWEEN ALUMINUM AND BROMINE
Elemental Br2 Is Reduced As Aluminum Is Oxidized.
The reaction is very exothermic and energy is given off in the form of light and heat: 2 Al (s) + 3 Br2 (l) --> 2 AlBr3 (s) or Al2Br6, Aluminum Tribromide.

PRECIPITATION: SILVER CHROMATE Ag2CrO4(s)
Sodium Chromate Added To Silver Nitrate
0.25M NaCrO4 (aq) is added to 0.10M AgNO3 (aq). There is more than enough silver nitrate to react with potassium chromate; silver nitrate is in excess. Silver chromate precipitates.

PHASES OF WATER: SOLID, LIQUID & GASEOUS
Boiling Water Reaches Gaseous State.
When the temperature rises above 100 deg. C., water turns to steam;, which rises & escapes from the beaker. At boiling point, bubbles of steam form in the liquid.

POTASSIUM REACTS EXPLOSIVELY WITH WATER
Elemental Potassium in Beaker of Water.
An exothermic displacement reaction between elemental Potassium and water producing aqueous Potassium Hydroxide and Hydrogen gas. Phenolphthalein indicator in the water turns pink as the solution becomes basic with the formation of Potassium Hydroxide.
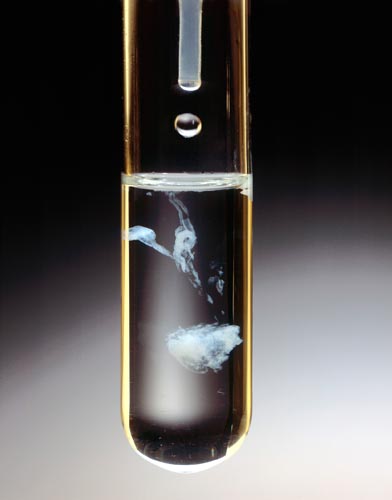
MAGNESIUM CHLORIDE ADDED TO TRISODIUM PHOSPHATE
Yields Precipitate of Magnesium Phosphate.
The addition of 0.5M MgCl2 (aq.) in the dropper to a 0.5M solution of Na3PO4 in the test tube forms a white solid Mg(PO4)2.

CELLULOID PING PONG BALL BURNS RAPIDLY
Nitrocellulose Products Are Highly Inflammable.
Cellulose mononitrate, cellulose dinitrate, and cellulose trinitrate are the forms of nitrocellulose, one of the first man made polymers. Because it was so unstable, camphor was added to amend the problem of detonation.

COPPER FLAME TEST (CuSO4)
Bright green flame indicates presence of copper.
(Transition Metal) Copper sulfate is dissociated by flame into gaseous atoms, not ions. Atoms of the element are raised to excited state by high temperature of flame. Excess energy from the atom is emitted as light of a characteristic wavelength.

ANTACID REACTS WITH VINEGAR
Calcium in Antacid Acts as Base, Neutralizes Acid
An antacid tablet is shown dissolving in white vinegar. The base in antacid neutralizes excess stomach acid, relieving heartburn and acid stomach.

IODINE SUBLIMATION DUE TO REACTION WITH ZINC
Zinc Mesh & Iodine Crystals Are Mixed In Test Tube
Adding water dissolves the zinc iodide which has formed on the surface of zinc allowing the reaction to continue. The reaction is exothermic causing the unreacted iodine to sublime quickly.

OXIDATION STATES OF VANADIUM - AMMONIUM VANADATE
Solution Of (NH4)VO4 Is Reduced With Zinc Amalgam.
Yellow acidified solution of (NH4)VO4, has vanadium in the +5 oxidation state as VO2 +. It is reduced with zinc amalgam: (Blue) In +4 oxidation state as VO 2+; (Green) In +3 oxidation state as V 3+; (Violet) In +2 oxidation state as V 2+.

CAPILLARY ACTION THRU CELERY STALK
Fluid absorption is evident in leaves of celery.
Surface tension causes the rise of water in a narrow tube. The water is pulled up until the surface tension force along the tube balances the weight of the water in the tube.

DECOMPOSITION OF RED MERCURIC OXIDE
HgO(s) Is Heated To A Temperature Of 400 degC
Mercuric oxide turns black and releases oxygen. Mercury begins to collect on the sides of the test tube.
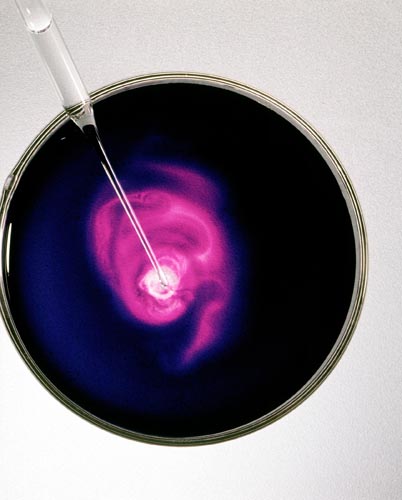
POTASSIUM PERMANGANATE: ADDING NaCl SOLUTION
Forms A Light Pink Solution Manganese Chloride II
Colorless salt solution added to KMnO4, a deep purple solution, reacts to form the light pink solution MnCl2.

PRECIPITATION: SILVER CHROMATE Ag2CrO4(s)
Potassium Chromate is in Microscale Pipette
0.25M K2CrO4 (aq) is added to 0.10M AgNO3 (aq). There is more than enough silver nitrate to react with potassium chromate; silver nitrate is in excess. Silver chromate precipitates.

CALCIUM FLAME TEST
Begins to burn
Calcium metal is held over Bunsen burner and begins to burn with orange flame.

CAPILLARY ACTION- A MOVEMENT OF WATER
Its Interaction With the Walls of A Thin Tube.
Water climbs up a thin glass tube because of the strong hydrogen-bonding interactions between the water and the oxygen at the surface of the glass (SiO2), climbing higher in the smaller diameter tube. Slant of tube doesn't affect height of the column.

DISTILLATION OF A SIMPLE MIXTURE
Collecting The Distillate
The distillation apparatus is used to separate a mixture of 2 liquids. The component with the lower boiling point vaporizes first, leaves the flask, converts back to liquid in the condenser & is collected in a beaker.

KIND OF OXYGEN BOND AFFECTS PHYSICAL STRUCTURE
Demonstrated By Quartz Crystal & Dry Ice.
Because of its simple molecular double bond, CO2 is a gas existing only as a volatile molecular solid at 78C or below. SiO2 is a giant covalent structure, with strong bonds in 3 dimensions, which make it a hard, high melting-point solid like quartz.

PHOSPHORUS PENTOXIDE ACIDIFIES WATER
Methyl Red Indicator with P4O10 (s) and Water.
P4O10(s) is stirred into the water forming phosphoric acid (indicator shifts to red) and generating enough heat to create steam in an exothermic reaction.. P4O10(s) + 6 H2O(l) --> 4 H3PO4(aq)

SUBLIMATION & RECRYSTALIZATION OF IODINE
Using Cold Finger Condenser.
When solid iodine is heated at ordinary atmospheric pressure, it sublimes (changes directly from solid to gas). When the I2 vapor comes in contact with a cold surface, it deposits I2 crystals.

CALCIUM HYDRIDE & WATER W/PHENOLPHTHALEIN
Calcium Hydride, CaH2(s) is added To Water;
Color turns red due to presence of OH- ions. The gas bubbles are H2.

TRIPLE POINT
Water In Solid, Liquid & Gas Form In Equilibrium
The point at which a combination of pressure and temperature allow a substance to coexist in all three phases (solid, liquid & gas) in equilibrium. The triple point temperature and pressure for water are 0.0098 degC and 6.0 x 10 -3 atm respectively.
















































































